
Principal Investigator
Microbiology
liuy@szbl.ac.cn
2022 - PresenShenzhen Bay Laboratory Junior Principal Investigator
2018-2021The University of Texas Medical Branch Postdoctoral Fellow
2013-2018 School of Life Science, Tsinghua University School of Life Science, Tsinghua University
2011-2013 School of Medicine, Tsinghua University Research Associate
2008-2011Shenzhen Beike Biotechnology Company Senior Research Assistant
2007-2008Department of Biology, The Chinese University of Hong Kong Research Assistant
2002-2006School of Life Science, Fudan University Bachelor of Science
1. Investigating the Genetic Evolution of Important Human Pathogenic Viruses.
2. The Pathogenesis of Severe Viral Diseases and the Molecular Mechanisms of Viral Infection and Transmission.
3. Developing Novel Viral Tools and Antiviral Strategies.
Dr. Yang Liu graduated with a bachelor's degree from Fudan University in 2006 and earned a Ph.D. in Biology from Tsinghua University in 2018. His research focuses on the genetic evolution, pathogenesis, and antiviral strategies of important human pathogenic viruses. He has identified several crucial factors affecting virus infection and transmission in dengue virus, Zika virus, and SARS-CoV-2 and has elucidated their genetic evolution patterns in nature.
During the COVID-19 pandemic, Dr. Yang Liu established various reverse genetics platforms for studying the novel coronavirus. He was the first to assess the impact of COVID-19 vaccines on neutralization capacity against multiple variants of SARS-CoV-2, providing vital information for the development and improvement of COVID-19 vaccine strategies. In 2022, Dr. Yang Liu joined the Infectious Disease Institute at Shenzhen Bay Laboratory as a principal investigator and obtained qualifications as a doctoral/master's supervisor at South China University of Technology/Sun Yat-sen University.
Upon his return to China, Dr. Yang Liu was honored with the Second Prize of the 2021 Chinese Medical Science and Technology Award (as the third author) and the First Prize of the 2022 Ministry of Education Science and Technology Award (as the fourth author). He secured the 2022 National Excellent Young Scientist Fund (Overseas) and numerous other national funding projects. His research achievements, published as the first author/corresponding author (including co-first/co-corresponding), have been featured in internationally renowned journals such as Nature, New England Journal of Medicine (NEJM), Cell, Nature Medicine, Nature Microbiology, Nature Communications, with a cumulative impact factor of 590 and over 4,500 citations.
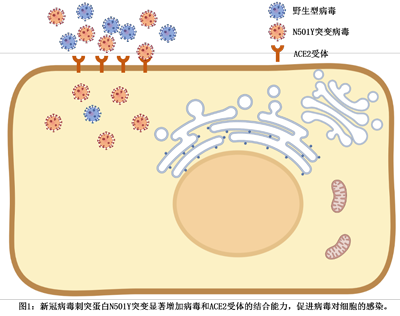
In this study, we revealed the amino acid mutation from Asparagine to Tyrosine at 501st position in the SARS-CoV-2 spike protein dramatically increased its binding affinity to ACE2 receptor, which benefit the viral infection and transmission. This explained why the SARS-CoV-2 became the dominant circulation lineages in the early 2021.

In this study, we established a recombinant SARS-CoV-2 mutant virus platform and made the world’s first step to assess the variation of SARS-CoV-2 vaccines’ protection ability against different SARS-CoV-2 variants. This study provided firsthand information for the improvement of future COVID-19 vaccines.
In this study, we found the amino acid substitution from Alanine to Valine at the 188th position of ZIKV NS1 protein promote the NS1 secretion in the host circulation system. The increased NS1 proteins were ingested by mosquito during its blood meal and inhibit the immune response in mosquito mid-gut, benefit the infection of ZIKV in mosquito. The number of ZIKV-infected mosquitoes increased in the environment and promote its transmission in nature. This study revealed the reason of ZIKV epidemic in the south Americas in 2017.
1. Liu Y, Liu J, Plante KS, Plante JA, Xie X, Zhang X, Ku Z, An Z, Scharton D, Schindewolf C, Menachery VD, Shi PY* and Weaver SC*. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature. 2022, 602: 294–299.
2. Liu Y, Zhang X, Liu J, Xia H, Zou J, Muruato AE, Periasamy S, Kurhade C, Plante JA, Bopp NE, Kalveram B, Bukreyev A, Ren P, Wang T, Menachery VD, Plante KS, Xie X*, Weaver SC* and Shi PY*. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions. Nature communications. 2022, 13: 4337
3. Liu Y, Liu J, Zou J, Kalveram B, Machado RR, Ren P, Türeli S, Smith DJ, Weaver, SC, Xie, X* and Shi PY*. Cross-neutralization and cross-protection among SARS-CoV-2 viruses bearing different variant spikes. Signal Transduction and Targeted Therapy. 2022, 7: 285.
4. Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Weaver SC, Muik A, Sahin U, Jansen KU, Xie X*, Dormitzer PR* and Shi PY*. Neutralizing activity of BNT162b2-elicited serum. New England Journal of Medicine. 2021, 384: 1466-1468.
5. Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, Zhang X, Muruato AE, Zou J, Fontes-Garfias CR, Mirchandani D, Scharton D, Bilello JP, Ku Z, An Z, Kalveram B, Freiberg AN, Menachery VD, Xie X*, Plante KS*, Weaver SC* and Shi PY*. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021, 592: 116-121.
6. Liu Y, Liu J, Xia H, Zhang X, Zou J, Fontes-Garfias CR, Weaver SC, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Muik A, Sahin U, Jansen KU, Xie X*, Dormitzer PR* and Shi PY*. BNT162b2-elicited neutralization against new SARS-CoV-2 spike variants. New England Journal of Medicine. 2021, P385: 472-474.
7. Zhang X, Liu Y, Liu J, Bailey AL, Plante KS, Plante JA, Zou J, Xia H, Bopp NE, Aguilar PV, Ren P, Menachery VD, Diamond MS, Weaver SC, Xie X* and Shi PY*. A trans-complementation system for SARS-CoV-2 recapitulates authentic viral replication without virulence. Cell. 2021, 184: 2229-2238.e13.
8. Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, Xia H, Swanson KA, Cutler M, Cooper D, Menachery VD, Weaver SC, Dormitzer PR* and Shi PY*. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nature Medicine. 2021, 27: 620-621.
9. Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, Li XF, Zhang R, Wang T, Qin CF, Wang P, Shi PY* and Cheng G*. Evolutionary Enhancement of Zika Virus Infectivity in Aedes Aegypti Mosquitoes. Nature. 2017, 545: 482.
10. Liu J, Liu Y, Nie K, Du S, Qiu J, Pang X, Wang P and Cheng G*. Flavivirus NS1 Protein in Infected Host Sera Enhances Viral Acquisition by Mosquitoes. Nature Microbiology. 2016, 1: 16087.