
Principal Investigator
Cell Biology
yincq@szbl.ac.cn
2020-PresentShenzhen Bay Laboratory Junior Principal Investigator
2020.04 - 2020.09 Massachusetts General Hospital Research Fellow
2016.10 - 2020.04Boston University Research Scientist
2016Drexel University PhD
2010 University of Science and Technology of China Bachelor
My research focuses on the regulation of signal transduction pathways mediated by protein post-translational modifications in malignant tumors such as melanoma, with an emphasis on exploring the mechanical role of signal transduction in driving the initiation and development of cancer, the discovery of new drug targets and the development of novel small molecule inhibitors.
To explore intervention opportunities for prevention and treatment of melanoma, I have conducted in-depth research on the initiation and development of melanoma in recent years, and have achieved a series of research accomplishments with important academic value and clinical translational potential. So far, I have published a total of 19 professional academic papers in research fields of signal transduction and melanoma biology, of which 6 were published as the first (including co-first) author in internationally renowned journals such as Cell, Nature, and Nature Communications. We explained the molecular mechanism of the G protein-coupled receptor MC1R being palmitoylated to suppress the occurrence of melanoma, and developed a pharmaceutical method to prevent the occurrence of melanoma (Nature, 2017). We also revealed serine/threonine kinase STK19 promotes the molecular mechanism of melanoma development through phosphorylation of NRAS, and developed a novel specific small molecule inhibitor that significantly inhibits the development of melanoma (Cell, 2019). I have applied for one U.S. patent and obtained two Chinese patent authorizations. Based on my previous research, I have won the Bristol-Myers Squibb Oncology Scholar-in-Training Award issued by the American Association for Cancer Research (AACR) in 2016, and received a research funding from the National Institutes of Health (NIH) as the sole principal investigator (PI) in 2020 as the K99/R00 NIH Pathway to Independence Award.

We explained the molecular mechanism of the G protein-coupled receptor MC1R being palmitoylated to suppress the occurrence of melanoma, and developed a pharmaceutical method to prevent the occurrence of melanoma (Nature, 2017).
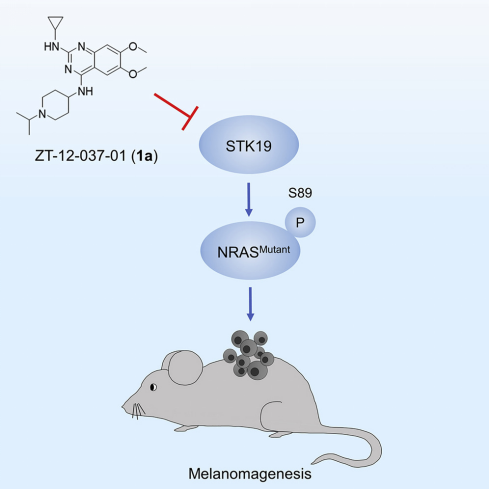
We revealed that serine/threonine kinase STK19 promotes the molecular mechanism of melanoma development through phosphorylation of NRAS, and developed a novel specific small molecule inhibitor that significantly inhibits the development of melanoma (Cell, 2019).
1. SLC25A1 and ACLY maintain cytosolic acetyl-CoA to regulate ferroptosis susceptibility via FSP1 acetylation. EMBO Journal. Li, W.#, Han, J.#, Huang, B. #, Xu, T. #, Wan, Y., Luo, D., Kong, W., Yu, Y., Zhang, L., Nian, Y. *, Chu, B. *, Yin, C. *2025, https://doi.org/10.1038/s44318-025-00369-5
2. PTPN23-dependent activation of PI3KC2α is a therapeutic vulnerability of BRAF-mutant cancers. Journal of Experimental Medicine. 222(3): e20241147. He, Y.#, Li, W.#, Zhang, M. #, Wang, H., Lin, P., Yu, Y., Huang, B., Hao, M., He, J., Kong, W., Luo, D., Xu, T., Wang, J., Huang, Y., Zhao, Q., Liu, Y., Zhang, J., Nian, Y., Zhang, L., Zhu, B., Yin, C.*2025/01. DOI:10.1084/jem.20241147
3. Palmitoylation-dependent regulation of GPX4 suppresses ferroptosis. Nature Communications. 16: 867. Huang, B.#, Wang, H.#, Liu, S.#, Hao, M.#, Luo, D., Zhou, Y., Huang, Y., Nian, Y., Zhang, L., Chu, B., Yin, C.*2025/01. DOI:10.1038/s41467-025-56344-5
4. Pharmacological targeting of casein kinase 1δ suppresses oncogenic NRAS-driven melanoma. Nature Communications. 15, 10088. Wen, Y.#, Wang, H.#, Yang, X.#, Zhu, Y., Li, M., Ma, X., Huang, L., Wan, R., Zhang, C., Li, S., Jia, H., Guo, Q., Lu, X., Li, Z., Shen, X., Zhang, Q.*, Si, L.*, Yin, C.*, Liu, T*. 2024/11.
5. Gut microbial metabolism in ferroptosis and colorectal cancer. Trends in Cell Biology. 10(11), Cui, W.#, Hao, M.#, Yang, X.*, Yin, C.*, Chu, B*. 2024/08.
6. Tryptophan metabolism acts as a new anti-ferroptotic pathway to mediate tumor growth. Advanced Science. 10(6), 2204006. Liu, D.#, Liang, C.#, Huang, B.#, Zhuang, X., Cui, W., Yang, L., Yang, Y., Zhang, Y., Fu, X., Zhang, X., Du, L., Gu, W., Wang, X., Yin, C.*, Chai, R.*, Chu, B.* 2023/02.
7. Aberrant promoter methylation of Wnt inhibitory factor-1 gene is a potential target for treating psoriasis. Clinical Immunology. 252023, 0, 109294. Liu, L. #, Zhou, Y. #, Luo, D. #, Sun, X., Li, H., Lu, Y., Wang, J., Zhang, M., Lin, N., Yin, C.*, Li, X.*, 2023/01.
8. AMPK phosphorylates ZDHHC13 to increase MC1R activity and suppress melanomagenesis. Cancer Research. 83 (7): 1062–1073. Sun, Y.#, Li, X.#, Yin, C.#, Zhang, J., Liang, E., Wu, X., Ni, Y., Arbesman, J., Goding, C. R., Chen, S. 2023/01
9. Pharmacological targeting of STK19 inhibits oncogenic NRAS-driven melanomagenesis. Cell. 176(5), 1113-1127. Yin, C.#, Zhu, B.#, Zhang, T.#, Liu, T.#, Chen, S., Liu, Y., Li, X., Miao, X., Li, S., Mi, X., Zhang, J., Li, L., Wei, G., Xu, Z., Gao, X., Huang, C., Wei, Z., Goding, C. R., Wang, P.*, Deng, X.*, Cui, R.* 2019/02
10. Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature. 549(7672), 399-403. Chen, S.#, Zhu, B.#, Yin, C.#, Liu, W., Han, C., Chen, B., Liu, T., Li, X., Chen, X., Li, C., Hu, L., Zhou, J., Xu, Z., Gao, X., Wu, X., Goding, C. R., Cui, R. 2017/09