
Principal Investigator
Cell biology
denglin@szbl.ac.cn
2022 - PresenShenzhen Bay Laboratory Junior Principal Investigator r
2015-2020Harvard Medical School & Dana-Farber Cancer Institute & Howard Hughes Medical Institute Research Fellow
2010-2015Dartmouth College Ph.D
2008-2010Northwest Agriculture & Forestry University M.S.
2004-2008Northwest Agriculture & Forestry University B. S.
The Deng laboratory is interested in cell cycle regulation and genome instability. We use a combination of cutting-edge techniques such as in vitro biochemical reconstitution, genome engineering, high throughput live-cell imaging, genome sequencing and so forth, to address how cells coordinate growth and division under normal and pathological conditions such as cancer.
Dr. Lin Deng has a long-standing interest in cell cycle regulation and genome instability. He obtained his Ph.D. in Biochemistry from Dartmouth College in U.S., supervised by Dr. James Moseley. His Ph.D. work dissected the signaling pathways for G2/M transition and discovered many novel factors and sub-cellular structures that regulate cell growth and division. Dr. Deng received his postdoc training at Harvard Medical School and Dana Farber Cancer Institute, jointly supervised by Dr. David Pellman and Joahnnes Walter. His postdoc research revealed novel mechanisms of genome instability induced by DNA replication stress and cell cycle defects.
Dr. Deng’s research has been published in peer-reviewed journals such as Molecular Cell, Current Biology, Molecular Biology of the Cell, Molecular and Cellular Biology, and so forth. These works have been recognized by several awards including The International Youth Friend of Shenzhen, China (2017), E. Lucile Smith Award for Excellence in Biochemistry, Dartmouth Medical School (2015), Chinese Government Award for Outstanding Students Abroad (2014), and John H. Copenhaver, Jr. and William H. Thomas, MD 1952 Fellow, Dartmouth College (2014).
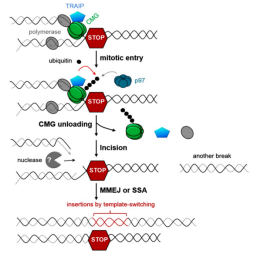
Mechanism of genome instability caused by premature mitosis
Representative papers:
Wang, J., Xiong, J., Zhang, S., Li, D., Chu, Q., Chang, W., Deng, L.,* and Ji, W.* A complex containing RhoBTB3-SHIP164-Vps26B promotes the biogenesis of early endosome buds at Golgi-endosome contacts. Cell Discovery, 2024, https://doi.org/10.1101/2022.09.30.510409
Chen, H., Wang, Z., Gong, L., Wang, Q., Chen, W., Wang, J., Ma, X., Ding, R., Li, X., Zou, X., Plass, M., Lian, C., Ni, T., Wei, G., Li, W.,* Deng, L.,* and Li, L.* A distinct class of pan-cancer susceptibility genes revealed by alternative polyadenylation transcriptome-wide association study. Nature Communications, 2024, 15, 1729
Chu, Q., Wang, J., Du, Y., Zhou, T., Shi, A., Xiong, J.*, Ji, W.*, and Deng, L.* Oligomeric CHMP7 mediates three-way ER junctions and ER-mitochondria interactions. Cell Death & Differentiation, 2022, doi:10.1038/s41418-022-01048-2 PMID:35962186.
Deng, L., Wu, R.A., Sonneville, R., Kochenova, O.V., Labib, K., Pellman, D., and Walter, J.C. Mitotic CDK promotes replisome disassembly, fork collapse, and complex DNA rearrangements. Molecular Cell, 2019, 73(5):915-929.e6. PMID: 30849395.
Deng, L., Lee, M.E. Schutt K.L., and Moseley, J.B. Phosphatases generate signal specificity downstream of Ssp1 kinase in fission yeast. Molecular and Cellular Biology, 2017, 37:e00494-16.doi: 10.1128/MCB.00494-16. PMID: 28223368.
Deng, L., Baldissard, S., Kettenbach, A.N., Gerber, S.A., and Moseley, J.B. Dueling kinases regulate cell size at division through the SAD kinase Cdr2. Current Biology, 2014, 24, 428-433. PMID: 24508166.
Deng, L., Kabeche, R., Wang, N., Wu, J.Q., and Moseley, J.B. Megadalton node assembly by binding of Skb1 to the membrane anchor Slf1. Molecular Biology of the Cell, 2014, 25, 2660-2668. PMID: 25009287.
Deng, L., and Moseley, J.B. Compartmentalized nodes control mitotic entry signaling in fission yeast. Molecular Biology of the Cell, 2013, 24, 1872-1881. PMID: 23615447.