
Principal Investigator
Microbiology
xinhaichen@szbl.ac.cn
2022 - PresenShezhen Bay Laboratory Junior Principal Investigator
2020-2022University of Chicago/HTRL Research Professional
2016-2020University of Chicago Postdoctoral Scholar
2015-2016Sun Yat-sen University Research Associate
2011-2015Sun Yat-sen University Ph.D.
2008-2011Fujian Agriculture and Forestry University M.S.
2004-2008Fujian Agriculture and Forestry University B.S.
(1) Exploring mechanisms of microbial immune evasion;
(2) Developing immunotherapies (including vaccines and mAbs) by targeting microbial immune evasion;
(3) Exploring how host impacts antibody therapy, and developing vaccine and mAb suitable for different groups of people.
(4) Combating antibiotic resistance
Dr. Xinhai Chen joined the Missiakas-Schneewind Laboratory in the Department of Microbiology at the University of Chicago for engaging the anti-infectious immunity. By integrating microbiology, immunology, vaccinology, and antibody engineering, Dr. Xinhai Chen is able to develop and optimize anti- Staphylococcus aureus vaccines and antibodies. He also investigates the mechanisms of action of antibodies and identifying host factors that support the activity of antibodies. Last four years, Dr. Xinhai Chen, as a first or corresponding author, has published three papers in PNAS and one paper in Journal of Infectious Diseases and Frontiers in Immunology. His patents related to antibody and vaccine against S. aureus are now actively pursuing clinical transformation in JANSSEN and IMMUNARTES.
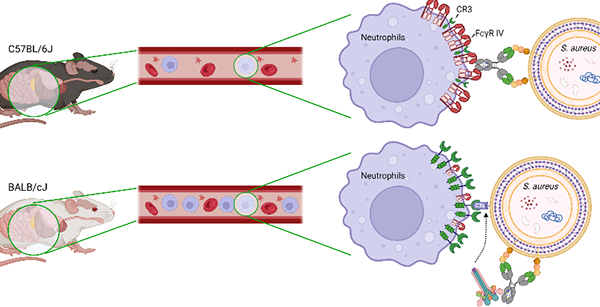
1. We find that protection with the same therapeutic antibody targeting S. aureus is achieved in an FcγR-dependent manner in C57BL/6J mice and CR3-dependent manner in BALB/cJ mice. Protection was associated with the preferential expression of FcγRIV on C57BL/6J neutrophils and CR3 on BALB/cJ neutrophils. Thus, both the A/I FcγRs ratio and the relative abundance of FcγRs over CRs impact the effector activity of an antibody and the mechanism of elimination of immune complexes.
2. Anti-S. aureus antibodies that could promote decolonization, prevent infection, or treat disease would alleviate the selection for drug resistance. The successful development of such antibodies is complicated by Staphylococcal protein A (SpA) in the envelope of S. aureus. SpA captures immunoglobulins via their constant region, preventing antibodies from initiating anti-staphylococcal activities. Here, we demonstrate that therapeutic anti-S. aureus antibodies can be engineered to avoid sequestration by SpA. Such antibodies display extended half-lives and improve bacterial uptake and killing by immune cells including macrophages and neutrophils.

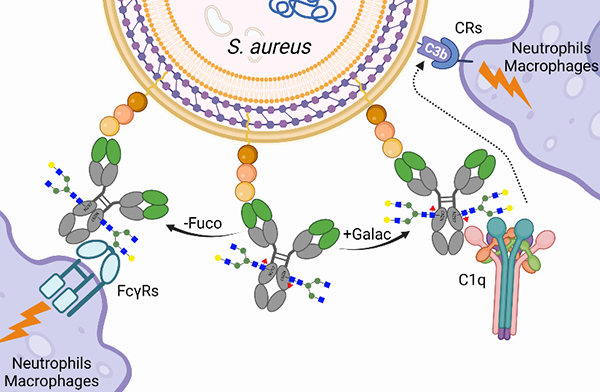
3. Recent studies have suggested that the glycosylation of antibody constant region is pivotal. We find that galactosylation of anti-S. aureus mAb that favors C1q recruitment is indispensable for opsonophagocytic killing of staphylococci and for protection against bloodstream infection in animals. The simple removal of fucosyl residues, which results in reduced C1q binding and increased engagement with FcγR, maintains the opsonophagocytic killing and protective attributes of the antibody. While the therapeutic benefit of monoclonal antibodies against infectious disease agents may be debatable, the functional characterization of such antibodies represents a powerful tool for the development of correlates of protection that may guide future vaccine trials.
Representative papers:
X Chen, H Gula, T Pius, C Ou, M Gomozkova, LX Wang, O Schneewind, D Missiakas*. Immunoglobulin G subclasses confer protection against Staphylococcus aureus bloodstream dissemination through distinct mechanisms in mouse models. PNAS, 2023, 120(14): e2220765120.
J Ye, X Chen*. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics, 2023; 12(1):67.
M Jiang, X Chen (Co-first author), H Li, XX Peng, B Peng*. Exogenous L-Alanine promotes phagocytosis of multidrug-resistant bacterial pathogens. EMBO Rep. 2023; 24(12): e49561.
X Chen, O Schneewind, D Missiakas*. Engineered human antibodies for the opsonization and killing of Staphylococcus aureus. PNAS, 2022, 119(4): e2114478119.
X Chen, M Shi, X Tong, HK Kim, LX Wang, O Schneewind, D Missiakas*. Glycosylation-dependent opsonophagocytic activity of Staphylococcal protein A antibodies. PNAS, 2020, 117(37): 22992-23000.
R Pang, H Zhou, Y Huang, Y Su*, X Chen*. Inhibition of host arginase activity against staphylococcal bloodstream infection by different metabolites. Front Immunol, 2020, 11, 1639.
X Chen, Y Sun, D Missiakas, O Schneewind*. Staphylococcus aureus decolonization of mice with monoclonal antibody neutralizing protein A. J Infect Dis, 2019, 219(6):884-888.