
Senior Principal Investigator
Neurobiology
chenz@szbl.ac.cn
Pleasant touch, itch, pain, instinctive behavior, emotion contagion, empathy, and prosocial behavior.
Dr. Chen has made a series of seminal contributions to our understanding of molecular and neural mechanisms involved in somatosensory transmission, including sensations such as itch and pleasant touch, from the skin to the brain. A leading pioneer in itch research, Dr. Chen went on to successfully identify the specific neuropeptide and spinal circuit responsible for pleasant touch. In recent years, he led the development of new behavioral assays to study instinctive social behaviors, notably contagious itching and social grooming. His research has radically transformed our view of how somatosensory modalities are encoded by neuropeptide signaling.
1. Discovery of itch-specific neuropeptides and spinal microcircuits.
Dr. Chen's flagship contribution is the groundbreaking discovery of itch-specific neuropeptides and spinal microcircuits. Itch is one of the most frequently encountered sensations in everyday life. For a long time it had been misinterpreted and overlooked by researchers, despite its negative impact on millions suffering from chronic itch worldwide and thus urgent need for effective medicine. In 2017, his discovery of the first itch-specific receptor, the Gastrin-Releasing Peptide Receptor (GRPR), and spinal circuit ignited tremendous enthusiasm across relevant fields and ushered forth a new era of itch research. Since then, his team has continued to illuminate the central mechanisms of different forms of itch, including histamine-dependent itch, opioid-related itch, mechanical itch, and contagious itch.
2. Discovery of pleasant touch-specific neuropeptide and spinal microcircuit
Our sense of touch is composed of discriminative (e.g., shape, pressure, and texture) and affective touch. Pleasant touch (e.g., hugging, licking, or massaging) subserves a plethora of functions, including soothing, pain-alleviation, sleep-promotion, social bonding, and attachment. Conversely, its deprivation has been shown to result in mental illness associated with psychiatric and neurodevelopmental conditions, such as autism spectrum disorders (ASD). Unfortunately, research regarding the molecular and neural mechanisms of pleasant touch had historically been stigmatized due to the lack of a clear, unbiased method to quantify the enjoyment mice receive from gentle touch.
In a major breakthrough, Dr. Chen's team identified prokineticin 2 (PROK2) as a pivotal neuropeptide and spinal microcircuit expressing PROKR2 for encoding and conveying pleasant touch information (Fig. 1). Mice lacking pleasant touch sensation failed to perform social grooming, a prosocial behavior critical to the normal development of animals. Critically, his team demonstrates that spinal PROKR2 neurons share hallmarks of human C tactile fibers that respond robustly to pleasant gentle touch, providing compelling evidence that mechanisms that sculpt the neural circuits of affective touch are conserved between humans and mice. These exciting findings highlight the central role that neuropeptide-mediate social grooming plays in animal well-being, and suggest that further study of pleasant touch in mice could have important implications for clinical research.
His studies indicate that neuropeptide-GPCR signaling is best suited for encoding somatosensory modalities with slow kinetics, while glutamate functions as a generic co-neurotransmitter that lacks modality specificity. Accordingly, Dr. Chen proposes a theoretic framework for encoding itch, inflammatory pain, and pleasant touch. This theory posits that sensory modalities with slow kinetics are encoded by neuropeptides with intrinsic modality specificity in sensory neurons, as well as by discrete spinal microcircuits defined by their respective GPCRs (Fig. 1).
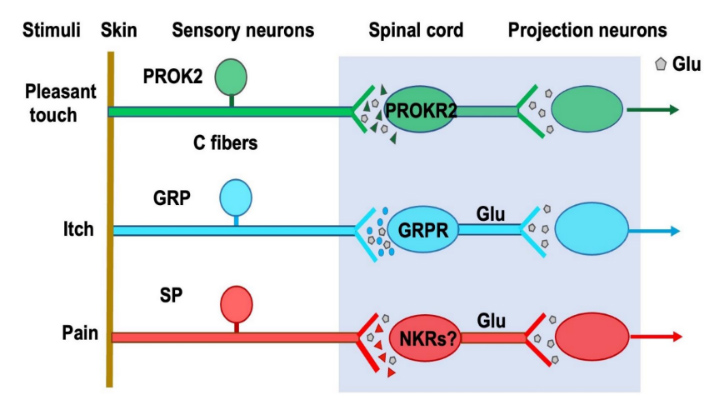
Fig 1. A neuropeptide code for itch, pain and pleasant touch. Somatosensory modalities with slow kinetics such as itch, pleasant touch and inflammatory pain are encoded by neuropeptides and their respective GPCRs in the spinal cord. Glu:glutamate, SP: substance P.
3. Discovery of a non-canonical visual pathway for relaying contagious itch information.
Contagious behavior is widespread the animal kingdom, and is crucial for social grouping, conformity, affiliation, and survival. Itch is known to be contagious in humans and primates. Dr. Chen's team pioneered a contagious itch test that, for the first time, permits a mechanistic elucidation of contagious behavior. He found that contagious itch behavior in mice is exclusively mediated by GRP-GRPR signaling in the suprachiasmatic nucleus (SCN), a circadian pacemaker. This surprising insight led to the discovery of a novel subcortical visual pathway — the retina-ipRGCs-SCN-PVT pathway — that relays contagious itch information (Fig. 2). Though the finding may not apply to humans, it indicates that the visual system in rodents is much more complex and evolutionarily ancient than previously thought. The discovery of contagious itch as a stress contagion rather than just motor mimicry also suggests that scratching behavior encodes salient social cues that inform conspecifics of adverse social environments. These unconventional findings can ultimately aid us in understanding the evolutionary history of prosocial behaviors like empathy and empathic helping behavior.
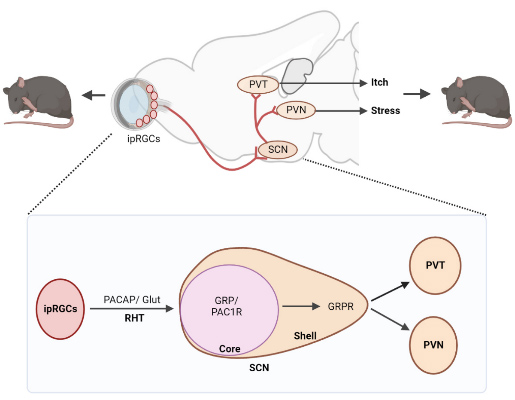
Fig 2. A non-canonical subcortical visual pathway for mediating contagious itch behavior and stress response. SCN: The suprachiasmatic nucleus. PVT: The paraventricular nucleus of the thalamus. PVN: The paraventricular nucleus of the hypothalamus. ipRGCs: intrinsic photosensitive retinal ganglion cells. RHT: The retinohypothalamic tract. PACAP: Pituitary adenylate-cyclase polypeptide. Glut: glutamate.
2023 - PresentSenior Principal Investigator, Shenzhen Bay Laboratory
2011 - 2023Director, Center for the Study of Itch and Sensory Disorders, Washington University School of Medicine, MO, USA
2000 - 2023Assistant Professor, Associate Professor and Professor, Washington University School of Medicine, MO, USA
1998 - 2000Senior Research Fellow, California Institute of Technology, CA, USA
1994 - 1998Postdoctoral Fellow, Howard Hughes Medical Institute, California Institute of Technology, CA, USA
1990 - 1994PhD in Genetics, University of Texas Health Science Center at Houston, USA
1983 - 1990Assistant Research Fellow, National Vaccine & Serum Institute, Beijing, China
1986 - 1987Visiting Scholar, London School of Hygiene & Tropical Medicine, London University, England
1979 - 1983Bachelor of Science in Virology, Wuhan University, China
2017 Russell D. and Mary B. Shelden Endowed Professor of Anesthesiology
# for corresponding author.
1.Liu, B., Qiao, L., Liu, K., Liu, J., Piccinni-Ash, T.J. and Chen, Z.F.#, 2022. Molecular and neural basis of pleasant touch sensation. Science, 376(6592), pp.483-491.
2.Gao, F., Ma, J., Yu, Y.Q., Gao, X.F., Bai, Y., Sun, Y., Liu, J., Liu, X., Barry, D.M., Wilhelm, S., Piccinni-Ash, T., Wang, N., Liu, D., Ross, R.A., Hao, Y., Huang, X., Jia, J.J., Yang, Q., Zheng, H., van Nispen, J., Chen, J., Li, H., Zhang, JY., Li YQ. and Chen, Z.F.#, 2022. A non-canonical retina-ipRGCs-SCN-PVT visual pathway for mediating contagious itch behavior. Cell Reports, 41(1).
3.Chen, Z.F.#, 2021. A neuropeptide code for itch. Nature Reviews
Neuroscience, 22(12), pp.758-776.
4.Barry, D.M., Liu, X.T., Liu, B., Liu, X.Y., Gao, F., Zeng, X., Liu, J., Yang, Q., Wilhelm, S., Yin, J., Tao, A. and Chen, Z.F.#, 2020. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nature Communications, 11(1), p.1397.
5.Chen, S., Gao, X.F., Zhou, Y., Liu, B.L., Liu, X.Y., Zhang, Y., Barry, D.M., Liu, K., Jiao, Y., Bardoni, R. and Yu, W. and Chen, Z.F.#, 2020. A spinal neural circuitry for converting touch to itch sensation. Nature Communications, 11(1), p.5074.
6.Yu, Y.Q., Barry, D.M., Hao, Y., Liu, X.T. and Chen, Z.F.#, 2017. Molecular and neural basis of contagious itch behavior in mice. Science, 355(6329), pp.1072-1076.
7.Zhao, Z.Q., Liu, X.Y., Jeffry, J., Karunarathne, W.A., Li, J.L., Munanairi, A., Zhou, X.Y., Li, H., Sun, Y.G., Wan, L. and Wu, Z.Y., Kim, S., Huo, FQ., MO, P., Barry, D.M., Zhang, C.K., Kim, J.Y., Gautam, N., Renner, K.J., Li, Y.Q. and Chen, Z.F.#, 2014. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron, 84(4), pp.821-834.
8.Liu, X.Y., Liu, Z.C., Sun, Y.G., Ross, M., Kim, S., Tsai, F.F., Li, Q.F., Jeffry, J., Kim, J.Y., Loh, H.H. and Chen, Z.F.#, 2011. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell, 147(2), pp.447-458.
9.Sun, Y.G., Zhao, Z.Q., Meng, X.L., Yin, J., Liu, X.Y. and Chen, Z.F.#, 2009. Cellular basis of itch sensation. Science, 325(5947), pp.1531-1534.
10.Sun, Y.G. and Chen, Z.F.#, 2007. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature, 448(7154), pp.700-703.
11.Ding, Y.Q., Marklund, U., Yuan, W., Yin, J., Wegman, L., Ericson, J., Deneris, E., Johnson, R.L. and Chen, Z.F.#, 2003. Lmx1b is essential for the development of serotonergic neurons. Nature Neuroscience, 6(9), pp.933-938.